Background
Degenerate eye disorders, such as glaucoma, cataracts and age-related macular degeneration (AMD), are prevalent causes of blindness and visual impairment worldwide. Other eye disorders, including limbal stem cell deficiency (LSCD), dry eye diseases (DED), and retinitis pigmentosa (RP), result in symptoms such as ocular discomfort and impaired visual function, significantly impacting quality of life. Traditional therapies are limited, primarily focus on delaying disease progression, while emerging stem cell therapy directly targets ocular tissues, aiming to restore ocular function by reconstructing ocular tissue.
Main text
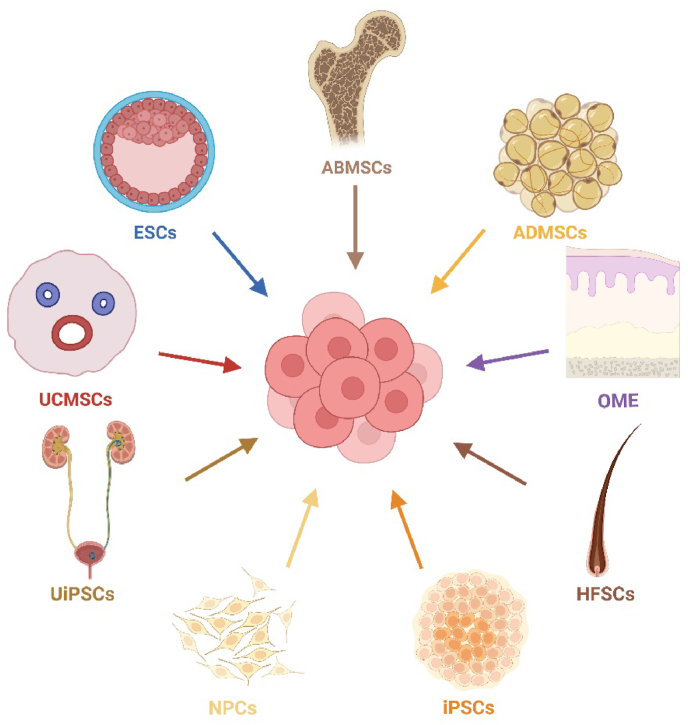
The utilization of stem cells for the treatment of diverse degenerative ocular diseases is becoming increasingly significant, owing to the regenerative and malleable properties of stem cells and their functional cells. Currently, stem cell therapy for ophthalmopathy involves various cell types, such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and retinal progenitor cells (RPCs). In the current article, we will review the current progress regarding the utilization of stem cells for the regeneration of ocular tissue covering key eye tissues from the cornea to the retina. These therapies aim to address the loss of functional cells, restore damaged ocular tissue and or in a paracrine-mediated manner. We also provide an overview of the ocular disorders that stem cell therapy is targeting, as well as the difficulties and opportunities in this field.
Conclusions
Stem cells can not only promote tissue regeneration but also release exosomes to mitigate inflammation and provide neuroprotection, making stem cell therapy emerge as a promising approach for treating a wide range of eye disorders through multiple mechanisms.
1. Introduction
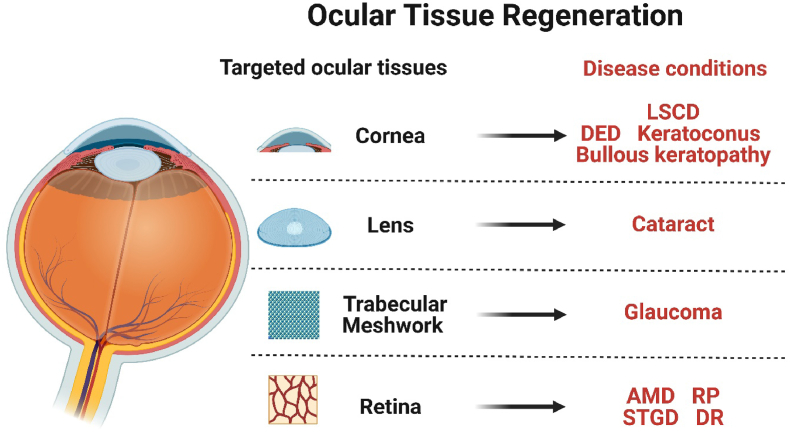
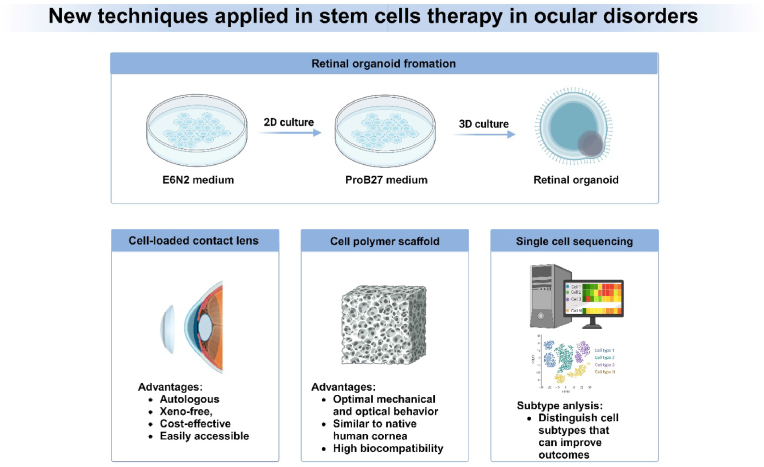
Degenerative eye disorders refer to a collection of irreversible diseases characterized by the gradual deterioration of ocular cells, leading to eventual cell death. Conventional therapies for degenerative eye disorders primarily aim to slow disease progression, but long-term benefits rely on the restoration and regeneration of damaged ocular tissue. Stem cells are undifferentiated cells that have not yet committed to a specific lineage.1 They have the ability to undergo division and can be induced to differentiate into various mature and functional cell types, which makes them a valuable tool. Stem cell therapy shows promise as a potential treatment for degenerative disorders, such as neurological disorders, heart diseases, and discogenic back pain, and thus significant efforts have been dedicated to its exploration. Numerous studies have demonstrated the potential of various cell types for regenerating ocular tissues, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and retinal progenitor cells (RPCs). Regarding the classification of clinical studies, most of the current studies are classified as phase I, with a primary focus on examining the adverse reactions and complications associated with the application of stem cell therapy (Fig. 1, Fig. 2). During the course of the investigation, various issues impede the advancement of the study. Non-autologous cell-based transplants pose a potential concern of rejection and may require recipients to undergo immune suppression. This obstacle can potentially be mitigated by utilizing cells sourced from closely related or human leukocyte antigen-matched individuals, or the patients themselves. In summary, this article provides an overview of the current advancements in utilizing stem cells for the regeneration of ocular tissue. Additionally, we explore the ocular disorders targeted by stem cell therapy and examine the perspectives and challenges within this field. Several ongoing/completed clinical trials are currently being conducted that make use of stem cells for treating human ocular diseases, as outlined in Table-1. We also summarize promising new techniques in the stem cell therapy in ocular disorders (Fig. 3).
Fig. 1.
Fig. 2.
Table 1.
Clinical trials registered on clinicaltrials.gov for treatment of different ocular diseases in last 5 years.
| NCT Number | Conditions | Study Title | Study Status | Phases | Enrollment | Start Date | Locations |
|---|---|---|---|---|---|---|---|
| NCT01691261 | Age Related Macular Degeneration | A Study Of Implantation Of Retinal Pigment Epithelium In Subjects With Acute Wet Age Related Macular Degeneration | Recruiting | Phase1 | 10 | 2021/10/14 | Moorfields Eye Hospital NHS Foundation Trust, London, EC1V 2PD, United Kingdom|Moorfields Eye Hospital NHS Foundation Trust, London, EC1V 2PD, United Kingdom |
| NCT03102138 | Age Related Macular Degeneration | Retinal Pigment Epithelium Safety Study For Patients In B4711001 | Not_yet_Recruiting | Unknown | 10 | 2024/1/21 | Moorfields Eye Hospital NHS Foundation Trust, 162 City Road, London, EC1V 2PD, United Kingdom |
| NCT04339764 | Age-Related Macular Degeneration | Autologous Transplantation of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Geographic Atrophy Associated With Age-Related Macular Degeneration | Recruiting | Phase1|Phase2 | 20 | 2020/9/23 | Johns Hopkins University, Baltimore, Maryland, 21205, United States|National Institutes of Health Clinical Center, Bethesda, Maryland, 20892, United States |
| NCT05187104 | Age-Related Macular Degeneration | Treatment of Age-related Macular Degeneration Using Retinal Stem and Progenitor Cells | Enrolling_by_invitation | Phase1|Phase2 | 20 | 2022/3/1 | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus, Minsk, 220072, Belarus |
| NCT05991986 | Age-Related Macular Degeneration | Preparation of Patient Autologous Induced Pluripotent Stem Cell-derived Retinal Cells for AMD | Not_yet_Recruiting | Unknown | 10 | 2024/8/23 | Unknown |
| NCT03981549 | Central Retinal Vein Occlusion | Treatment of Central Retinal Vein Occlusion Using Stem Cells Study | Active_Not_Recruiting | Phase1|Phase2 | 16 | 2019/10/22 | Department of Ophthalmology & Vision Science, University of California Davis Eye Center, Sacramento, California, 95817, United States |
| NCT03990051 | Chronic Ocular Graft-versus-host Disease | Treatment Safety and Efficacy of Pro-ocularTM 1% for Chronic Ocular Graft Following Allogeneic HSCT. | Completed | Phase2 | 33 | 2019/10/17 | Massachusetts Eye and Ear Longwood, Boston, Massachusetts, 02115, United States |
| NCT04932629 | Corneal Scars and Opacities | To Evaluate the Clinical Safety and Efficacy of Limbal Stem Cell for Treatment of Superficial Corneal Pathologies”. | Unknown | Early_Phase1 | 20 | 2024/7/21 | Unknown |
| NCT05705024 | Corneal Ulcer | Efficacy of Locally Delivered Allogeneic Mesenchymal Stromal Cells | Recruiting | Phase2 | 38 | 2023/9/29 | Department of Ophthalmology and Visual Sciences, Chicago, Illinois, 60612, United States|University of Maryland at Baltimore, Baltimore, Maryland, 21201, United States|Mass Eye and Ear Infirmary, Boston, Massachusetts, 02114, United States|University of Pennsylvania, Scheie Eye Institute, Philadelphia, Pennsylvania, 19104, United States |
| NCT04627428 | Dry Age-related Macular Degeneration | Safety and Tolerability of RPE Stem Cell-derived RPE(RPESC-RPE) Transplantation in Patients With Dry Age-related Macular Degeneration (AMD) | Recruiting | Phase1|Phase2 | 18 | 2022/4/5 | University of Michigan Kellogg Eye Center, Ann Arbor, Michigan, 48105, United States |
| NCT04213248 | Dry Eye | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | Recruiting | Phase1|Phase2 | 27 | 2020/2/21 | Zhongshan Ophthalmic Center, Guangzhou, Guangdong, 510000, China |
| NCT05738629 | Dry Eye Disease | Safety and Efficacy of Pluripotent Stem Cell-derived Mesenchymal Stem Cell Exosome (PSC-MSC-Exo) Eye Drops Treatment for Dry Eye Diseases Post RefrActive Surgery and Associated With Blepharospasm | Not_yet_Recruiting | Phase1|Phase2 | 12 | 2024/3/23 | 2nd Affiliated Hospital, School of Medicine, Zhejiang University, Hanzhou, Zhejiang, 310000, China |
| NCT03302273 | Dry Eye Syndromes|Dry Eye|Ocular Inflammation|Ocular Surface Disease|Ocular Discomfort|Blepharitis | Corneal Epithelial Stem Cells and Dry Eye Disease | Completed | Unknown | 17 | 2019/2/1 | Rush Eye Associates, Amarillo, Texas, 79109, United States |
| NCT03878628 | Dry Eye|Kerato Conjunctivitis Sicca|Aqueous Tear Deficiency | Treatment With Allogeneic Adipose-derived Mesenchymal Stem Cells in Patients With Aqueous Deficient Dry Eye Disease | Completed | Early_Phase1 | 7 | 2019/10/16 | Rigshospitalet, Copenhagen, DK, 2200, Denmark |
| NCT05147701 | Eye Diseases|Retinitis Pigmentosa|Glaucoma|Diabetic Retinopathy|Macular Degeneration|Traumatic Optic Neuropathy|Optic Atrophy | Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Eye Diseases | Recruiting | Phase1 | 20 | 2022/2/1 | Medical Surgical Associates Center, St. John’s, Antigua and Barbuda|Center for Investigation in Tissue Engineering and Cellular Therapy, Buenos Aires, Argentina|Medyca Bosques, San Pedro Garza García, N.L, Mexico |
| NCT05170347 | Graft Vs Host Disease|Haematological Malignancy|Cancer | oGVHD After Bone Marrow Transplantation: a Territory-wide Cohort | Recruiting | Unknown | 500 | 2021/4/30 | Department of Ophthalmology, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong |
| NCT04792580 | Graft-versus-host-disease|Ocular Graft-versus-host Disease | The Effects and Safety of 5% Lifitegrast Ophthalmic Solution in Subjects With Dry Eye Disease in Ocular Graft-versus-Host Disease | Recruiting | Early_Phase1 | 30 | 2022/10/22 | Richard W Yee, MD PLLC, Bellaire, Texas, 77401, United States |
| NCT04615455 | Keratoconjunctivitis Sicca, in Sjogren’s Syndrome | Mesenchymal Stem Cell Therapy of Dry Eye Disease in Patients With sjǒgren’s Syndrome | Completed | Phase2 | 40 | 2020/11/3 | Rigshospitalet, Copenhagen, DK, 2200, Denmark |
| NCT04594512 | Keratoconus|Keratoconus of Right Eye|Keratoconus, Unstable, Right Eye | Fresh Corneal Lenticule Implantation and Autologous Serum – Case Report | Active_Not_Recruiting | Unknown | 1 | 2019/5/8 | Eye Hospital Pristina, Pristina, 10000, Kosovo |
| NCT04636918 | Leukemia (Both ALL and AML)|MDS-EB-1 | Ikervis for DED Due to GVHD Post Allo-HSCT | Unknown | Phase4 | 40 | 2019/11/28 | Singapore Eye Research Institute, Singapore, Singapore |
| NCT03884569 | Limbal Stem-cell Deficiency | Cultivated Limbal Epithelial Transplantation (CLET) for Limbal Stem Cell Deficiency (LSCD) | Not_yet_Recruiting | Unknown | 30 | 2022/4/1 | IOBA, Valladolid, 47011, Spain |
| NCT03957954 | Limbal Stem-cell Deficiency | Stem Cell Therapy for Limbal Stem Cell Deficiency | Recruiting | Phase1 | 20 | 2020/9/30 | University of California, Los Angeles, California, 90095, United States |
| NCT04995926 | Limbal Stem-cell Deficiency | Labial Mucosal Epithelium Grafting for Corneal Limbus Substitution | Recruiting | Unknown | 20 | 2021/7/1 | The S. Fyodorov Eye Microsurgery Federal State Institution, Moscow, 127473, Russian Federation |
| NCT05909735 | Limbal Stem-cell Deficiency|Congenital Aniridia | Treatment of LSCD With DM | Recruiting | Phase1 | 20 | 2023/11/30 | University of Minnesota, Minneapolis, Minnesota, 55455, United States |
| NCT04773431 | Limbus Corneae|Limbus Corneae Insufficiency Syndrome | Safety Evaluation of LSCD101 Transplantation for Limbal Stem Cell Deficiency | Completed | Phase1 | 6 | 2020/1/31 | CliPS, Seoul, 04168, Korea, Republic of |
| NCT04642729 | Macular Corneal Dystrophy | Fresh Corneal Lenticule Implantation in Macular Corneal Distrophy With Relex Smile Surgery | Enrolling_by_invitation | Unknown | 15 | 2019/6/1 | Eye Hospital Pristina, Pristina, 10000, Kosovo |
| NCT05445063 | Macular Degeneration | Safety and Efficacy of Autologous Transplantation of iPSC-RPE in the Treatment of Macular Degeneration | Recruiting | Phase1 | 10 | 2024/8/22 | Beijing Tongren Hospitol, Capital Medical University, Beijing, Beijing, 100730, China |
| NCT05784519 | Mesenchymal Stem Cell|Dry Eye Syndromes | Therapeutic Effect of Stem Cell Eye Drops on Dry Eye Disease | Not_yet_Recruiting | Early_Phase1 | 10 | 2023/6/1 | Unknown |
| NCT04877067 | Methanol Poisoning|Toxic Optic Neuropathy|Stem Cell Tyrosine Kinase 1 Y842X|Magnetic Field Exposure | Therapy of Toxic Optic Neuropathy Via Combination of Stem Cells With Electromagnetic Stimulation | Completed | Phase3 | 18 | 2019/4/1 | Ankara University Biotechnology Institute, Ankara, Türkiye, 06312, Türkiye|Umut Arslan, Ankara, 06000, Türkiye |
| NCT05658237 | Myopic Chorioretinal Atrophy | Clinical Study of PAL-222 Targeting Patients With Myopic Chorioretinal Atrophy (PAMyCA) | Recruiting | Unknown | 10 | 2023/2/17 | Nagoya city university hospital, Nagoya, Aichi, Japan |
| NCT03829566 | Neuromyelitis Optica|Devic’s Disease|NMO Spectrum Disorder | Autologous Transplant To End NMO Spectrum Disorder | Withdrawn | Phase2|Phase3 | 0 | 2024/11/19 | Northwestern University, Feinberg School of Medicine, Chicago, Illinois, 60611, United States|Northwestern University, Chicago, Illinois, 60611, United States |
| NCT04552730 | Neurotrophic Keratitis | Nerve Growth Factor for the Treatment of Cornea Disease | Completed | Unknown | 5 | 2020/10/14 | Stanford University, Stanford, California, 94305, United States |
| NCT05311514 | Ocular Graft-versus-host Disease | Allogeneic Platelet Lysate Eye Drops for the Treatment of Severe Chronic Ocular Graft-versus-host Disease | Recruiting | Phase2 | 30 | 2021/4/1 | First Pavlov State Medical University of St. Petersburg, Saint-Petersburg, 197089, Russian Federation |
| NCT05279157 | Ophthalmological Disorder|Corneal Dystrophy|Treatment|Therapy|Keratoconus | Autologous Adipose-Derived Adult Stem Cell Implantation for Corneal Diseases (ADASCs-CT-CD) | Completed | Phase2 | 15 | 2022/4/19 | Jorge L. Alio, Alicante, 03016, Spain |
| NCT06200727 | Platelet-rich Fibrin|Macular Holes|Pterygium|Glaucoma | Platelet-rich Fibrin(PRF) Membrane in Ophthalmic Diseases | Active_Not_Recruiting | Unknown | 170 | 2023/1/1 | Eye Center, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, China |
| NCT05528809 | Primary Gougerot-sjǒgren Syndrome|Systemic Sclerosis, Diffuse | Quantification and Characterization of Circulating Epithelial and Endothelial Cells in Gougerot-sjǒgren Syndrome, Compared to Systemic Sclerosis | Recruiting | Unknown | 40 | 2022/10/12 | Montpellier University Hospital, Montpellier, France |
| NCT04490876 | Proliferative Vitreoretinopathy | Outcomes of Extensive Brilliant Blue G-Assisted Internal Limiting Membrane Peeling in Proliferative Vitreoretinopathy | Completed | Unknown | 14 | 2020/7/20 | Centro Oftalmologico Dr Charles, Buenos Aires, Capital Federal, 1116, Argentina |
| NCT03944239 | Retinitis Pigmentosa | Safety and Efficacy of Subretinal Transplantation of Clinical Human Embryonic Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Retinitis Pigmentosa | Unknown | Phase1 | 10 | 2024/5/20 | Beijing Tongren Hospitol, Capital Medical University, Beijing, Beijing, 100730, China |
| NCT04284293 | Retinitis Pigmentosa | CNS10-NPC for the Treatment of RP | Recruiting | Phase1 | 16 | 2021/7/22 | Retina-Vitreous Associates Medical Group, Beverly Hills, California, 90211, United States |
| NCT04604899 | Retinitis Pigmentosa | Safety of Repeat Intravitreal Injection of Human Retinal Progenitor Cells (jCell) in Adult Subjects With Retinitis Pigmentosa | Completed | Phase2 | 30 | 2020/12/1 | Gavin Herbert Eye Inst, Univ Cal Irvine, Irvine, California, 92697, United States|Retina-Vitreous Associates Medical Group, Los Angeles, California, 90074, United States|Ophthalmic Consultants of Boston, Boston, Massachusetts, 02114, United States |
| NCT04925687 | Retinitis Pigmentosa | Phase 1 Study of Intravitreal Autologous CD34+ Stem Cell Therapy for Retinitis Pigmentosa | Active_Not_Recruiting | Phase1 | 4 | 2021/6/1 | University of California Davis, Sacramento, California, 95817, United States |
| NCT05413148 | Retinitis Pigmentosa | The Effect of Stem Cells and Stem Cell Exosomes on Visual Functions in Patients With Retinitis Pigmentosa | Recruiting | Phase2|Phase3 | 135 | 2022/8/5 | Erciyes University, Faculty of Medicine, Kayseri, 38039, Türkiye |
| NCT05786287 | Retinitis Pigmentosa | Long-term Safety of UC-MSC Transplantation in Patients With Retinitis Pigmentosa | Enrolling_by_invitation | Unknown | 18 | 2023/7/1 | Jakarta Eye Center Hospital, Jakarta, DKI Jakarta, Indonesia|Sardjito Hospital, Yogyakarta, Special Region, 55284, Indonesia |
| NCT05909488 | Retinitis Pigmentosa | Role of UC-MSC and CM to Inhibit Vision Loss in Retinitis Pigmentosa Phase I/II | Recruiting | Phase2|Phase3 | 30 | 2023/9/1 | RSUP Dr. Sardjito, Yogyakarta, DI Yogyakarta, 55284, Indonesia |
| NCT04763369 | Retinitis Pigmentosa (RP) | Investigation of Therapeutic Efficacy and Safety of UMSCs for the Management of Retinitis Pigmentosa (RP) | Unknown | Phase2 | 50 | 2024/2/21 | Stem Cell laboratory, Jinnah Burn & Reconstructive Surgery Centre, Lahore, Punjab, 54550, Pakistan |
| NCT05494671 | Stem Cell Transplantation | Studying Corneal Epithelial Stability Following Limbal Stem Cell Transplantation in Cases of Limbal Stem Cell Deficiency | Active_Not_Recruiting | Unknown | 12 | 2022/1/1 | Kasr Alainy, Cairo, 11562, Egypt |
| NCT03949881 | Total Bilateral Limbal Cell Deficiency | Cultured Autologous Oral Mucosa Epithelial Sheet for the Treatment of Bilateral Limbal Stem Cell Deficiency | Recruiting | Phase1|Phase2 | 40 | 2020/11/13 | Ophthalmology department, Edouard Herriot hospital, Hospices Civils de Lyon, Lyon, 69003, France |
| NCT05047276 | Uveal Melanoma, Metastatic | Phase I/II Study of AloCelyvir in Patients With Metastatic Uveal Melanoma | Not_yet_Recruiting | Phase1|Phase2 | 16 | 2021/10/1 | ICO Hospitalet, Hospitalet de Llobregat, Barcelona, 08908, Spain |
| NCT05607095 | Uveal Melanoma|Melanoma|Metastatic Uveal Melanoma|Metastatic Melanoma | Pilot Trial of Autologous Tumor Infiltrating Lymphocytes (LN-144) for Patients With Metastatic Uveal Melanoma | Recruiting | Phase1 | 10 | 2022/11/1 | Memorial Sloan Kettering Westchester, Harrison, New York, 10604, United States|Memorial Sloan Kettering Cancer Center (All Protocol Activities), New York, New York, 10065, United States |
Fig. 3.
2. Applications of cornea regeneration in ophthalmic disease treatment
2.1. Regenerative treatment for limbal stem cell deficiency (LSCD)
Limbal stem cells (LSCs) are a type of adult stem cells located at the limbus, which possess the ability to differentiate into corneal epithelium. limbal stem cell deficiency (LSCD) occurs when there is inadequate or impaired activity of LSCs.2 Individuals with LSCD frequently exhibit symptoms including reduced vision, dryness, redness, irritation, photophobia, blepharospasm, symblepharon, and pain. Initially, they may remain asymptomatic.3
Since the groundbreaking work of Kenyon and Tseng in 1989,4 when they pioneered ocular stem cell therapy through limbal tissue transplantation, the field has witnessed tremendous growth. Various sources for tissue transplantation have been explored, including cultivated limbal epithelium, conjunctival epithelium, oral mucosal epithelium (OME), dental pulp stem cells (DPSCs), hair follicle-derived stem cells (HFSCs), human ESCs, and iPSCs.
2.1.1. Source from cultivated limbal epithelium
Treatment for LSCD primarily relies on limbal autografting techniques, including conjunctival limbal autografting (CLAU), cultivated limbal epithelium transplantation (CLET), and simple limbal epithelial transplantation (SLET).5
CLAU, first described by Kenyon and Tseng in 1989, is associated with various complications such as infection, scarring, and chronic inflammation due to the large graft area.6 To improve treatment outcomes and mitigate risks, several improvements, including CLET and SLET, have been developed utilizing either autologous or allogenic tissue. Reports have shown that CLET requires a smaller dose of donor’s limbal epithelium, reducing the risk of complications, and favorable results have been reported.7, 8, 9, 10 Some researchers reported that autologous CLET has high long-term survival rates and greater safety compared to allogenic CLET.11 In some cases of partial LSCD where stem cell transplantation may not be necessary, Sharma et al. have suggested the use of amniotic membrane transplantation (AMT) alone as a therapeutic modality, which has comparable curative effect to cultivated LSCs transplantation.12 Sangwan et al. introduced a novel surgical technique called SLET, involving the extraction of a small biopsy of healthy eye limbal tissue. This tissue is then divided into 8–10 pieces and placed on fresh human amniotic membrane adhered to the diseased eye’s scraped corneal bed. SLET simplifies the procedure as cell expansion occurs in vivo, reducing treatment time and costs.13 An ongoing clinical trial (ISRCTN12217540) is comparing the effectiveness of CLET and SLET in treating LSCD in ocular burn patients. Another innovative approach involves the transplantation of LSCs cultured on the concave surface of a siloxane-hydrogel extended-wear contact lens, which has shown promising results. The contact lens approach offers the advantages of being autologous, xeno-free, cost-effective, and easily accessible.14 Additionally, Miguel et al. have developed a tissue-engineered scaffold using a fibrin and agarose mixture, where corneal epithelial cells, corneal fibroblasts, and corneal endothelial cells are co-cultured.15 These co-cultured fibrin-agarose scaffolds exhibit optimal mechanical and optical behavior similar to native human corneas, and have demonstrated high biocompatibility in animal models.16
2.1.2. Source from cultivated conjunctival epithelium
Besides the corneal epithelium, the conjunctival epithelium can be used as an alternative source of cells for ocular surface reconstruction. Conjunctival epithelium and corneal epithelial cells are the most similar stratified epithelial tissues that exist in the body.17 Patients with a range of ocular surface problems have had successful conjunctival regeneration through the transplantation of autologous conjunctival epithelial cells that are cultivated outside the body.18,19 With positive results noted, this method has also been applied to corneal surface reconstruction in patients with LSCD.20 In summary, the conjunctival epithelium, with its close resemblance to corneal epithelial cells, offers a promising cell source for ocular surface reconstruction.
2.1.3. Source from other stem cell resources
According to current knowledge, the pool of stem cells capable of regenerating corneal tissue for the treatment of LSCD encompasses OME, DPSC, HFSC, iPSC, and ESC. OME, a non-limbal cell type utilized for therapeutic purposes, has gained broad application in the treatment of LSCD, with researchers worldwide reporting encouraging outcomes.21, 22, 23, 24, 25, 26, 27 Instances of Stevens-Johnson syndrome, having an immunological basis and potentially affecting the oral mucosa, are included.28 DPSCs share important characteristics with LSCs in the eye and can be administered via a contact lens.29 The current study provides evidence that HFSCs can differentiate into a phenotype phenotypically similar to corneal epithelial cells for reconstructing the ocular surface in LSCD mice.30 Owing to uncontrolled proliferation, non-specific differentiation, transplantation rejection, tumorigenesis, and ethical concerns, clinical trials applying ESCs as grafts have several limitations. Previous studies have demonstrated different strategies for corneal epithelial cell differentiation from human iPSCs.31,32 Nevertheless, there is a lack of reported clinical studies on the use of iPSCs for treating LSCD.
2.2. Regenerative treatment for dry eye diseases (DED)
Impaired tear film homeostasis and ocular symptoms are the hallmarks of dry eye diseases (DED), a multifactorial illness of the ocular surface.33 Common symptoms of DED are ocular discomfort and blurred vision, which adversely affect visual function and quality of life. MSCs are multipotent stem cells that have been shown to reduce inflammation and enhance tissue repair when transplanted in vivo. Studies have demonstrated that injecting a product consisting of allogeneic adipose-derived MSCs (ADMSCs) to the lacrimal gland is a safe and feasible treatment for severe DED with aqueous deficiency.34 An extremely dangerous long-term side effect of allogeneic hematopoietic stem cell transplantation is chronic Graft-versus-Host Disease (cGVHD).35 Inflammation and fibrosis in patients with cGVHD often result in severe dry eye syndrome. Patients’ symptoms improved following intravenous injection of MSCs, resulting in improved dry eye symptoms and Schirmer test results.36 Administering exosomes derived from MSCs (MSC-exo) as eye drops significantly alleviate dry eye disease associated with cGVHD.37
2.3. Regenerative treatment for keratoconus
Keratoconus is a progressive eye condition characterized by corneal thinning, protrusion, and distortion, leading to vision loss caused by irregular astigmatism.38 Traditional treatments for keratoconus involve corneal transplantation techniques, such as penetrating or lamellar transplantation. However, these methods have limitations, including graft rejection, slow visual recovery,38 and limited availability of donor corneal tissue.39 Tissue engineering of the corneal stroma aims to overcome these challenges but is currently hindered by the complex structure of the cornea.40 Stem cell therapy for corneal stroma has gained attention as a potential solution as stem cells can not only prolifrate and differentiate into mature corneal cells, but also secret new collagen within the corneal stroma.41,42
To our current understanding, the sole published clinical data pertaining to the regeneration and therapeutic application of stem cells in the human corneal stroma originates from an earlier investigation conducted by Alio et al.43, 44, 45, 46 In their research, patients diagnosed with keratoconus were divided into three experimental groups, with individuals receiving transplantation of autologous adipose-derived adult stem cells (ADASCs), transplantation of decellularized corneal stromal lamina, and transplantation of recellularized lamina containing ADASCs, respectively.47 Larger sample numbers and additional study are required to confirm the effectiveness of these treatments.
2.4. Regenerative treatment for bullous keratopathy
Bullous keratopathy is a corneal disease resulting from endothelial decompensation. Patients with bullous keratopathy may experience impaired vision and ocular discomfort due to epithelial damage and stromal edema. Occasionally, visual acuity following corneal transplantation may be unsatisfactory due to irregularities in the corneal structure induced by surgery. Corneal endothelial cells (CECs) were successfully utilized in an initial clinical trial involving 11 patients diagnosed with bullous keratopathy and exhibiting a deficiency of detectable CECs.48 Cells passaged from human corneal endothelium and supplemented with a rho-associated protein kinase (ROCK) inhibitor were injected into the chosen treatment eye’s anterior chamber. After 24 weeks post-injection, the transparency of the cornea was restored, and there was an observed rise in CEC density.
3. Applications of lens regeneration in ophthalmic disease treatment
3.1. Regenerative treatment for cataracts
In the mature lens, lens epithelial stem/progenitor cells (LECs) cover the anterior surface of the lens and initiate differentiation into lens fibers at the equatorial region. LECs play crucial roles in sustaining self-renewal and protecting against external injuries, highlighting their important functions.49 Lin et al.50 demonstrated a significant 11-fold increase in LEC numbers following the complete surgical removal of lens contents while preserving the empty capsular bag scaffold, indicative of the robust regenerative capability of human LECs after injury. Nevertheless, the disordered proliferation of residual LECs subsequent to surgery can result in the development of visual axis opacification (VAO), thereby increasing the risk of cataracts, a primary cause of global blindness. With the present approach to pediatric cataract surgery, the emergence of visually significant posterior capsule opacification is anticipated in almost all patients within weeks or months following the surgical procedure, attributable to the abnormal proliferation of residual LECs. To mitigate the occurrence of VAO, Lin and colleagues developed and implemented a novel capsulorhexis technique during pediatric cataract surgery to safeguard the regenerative capacity of LECs.50 Distinguishing it from the conventional approach for pediatric cataract surgery, Lin’s method substantially decreases wound size and relocates the capsulorhexis opening from the central visual axis to the periphery. Findings from the clinical trial demonstrated the functionality of the regenerated lenses and a remarkable 20-fold reduction in complications, including VAO, endorsing the superior efficacy and safety of the innovative treatment. Substantial advancements have been achieved in the investigation of exogenous LSCs. Yao and colleagues51 successfully utilized induced lentoid bodies (LBs) derived from urinary human induced pluripotent stem cells (UiPSCs) to create an in vitro model of congenital cataract. This model provides a robust platform for studying the pathogenesis of cataract and screening potential drug candidates for cataract treatment. Furthermore, Yao and colleagues52 successfully achieved in situ lens regeneration using cells derived from human embryonic stem cells (hESCs), resulting in the thickest and most transparent lens documented thus far. They also demonstrated the participant of the Wnt/PCP pathway in the process of lens regeneration, which offers novel therapeutic approaches and insights into cataract development.
4. Applications of trabecular meshwork regeneration application in ophthalmic disease treatment
4.1. Regenerative treatment for glaucoma
Primary open-angle glaucoma (POAG) is the primary cause of irreversible blindness, affecting over 70 million individuals globally. Glaucoma is a degenerative disease that leads to severe headache and visual loss due to the progressive degeneration of retinal ganglion cells. Maintaining a precise intraocular pressure (IOP) is the responsibility of the trabecular meshwork (TM), which offers resistance to the outflow of aqueous humor. In contrast to traditional treatments, stem cell therapy directly targets the TM, facilitating its repopulation and function restoration. Trabecular meshwork stem cells (TMSCs) have shown the ability to actively migrate to the TM region and differentiate into TM cells when injected into the anterior chamber of a normal mouse.53 Moreover, studies have demonstrated that iPSC-derived TM cells restored TM function associated with IOP in ex vivo experiments using human anterior segments54 and in in vivo glaucoma mouse models.55, 56, 57, 58 Additionally, injecting MSCs into the ocular anterior chamber has a neuroprotective effect in glaucoma, which is comparable to the effect of TMSCs.59,60 To ensure targeted delivery of stem cells to the TM region, magnetic nanoparticles were employed for labeling MSCs, enabling their magnetic steering towards the TM after injection into the anterior chamber of mouse models’ eyes. This approach demonstrated high efficiency, preserving stem cell viability and multipotency.61 Sara et al. proposed a novel phase I clinical study to investigate the regeneration of the TM.62 Based on their description, ADMSCs were recommended for human clinical trials due to their potential to reduce the risk of immune rejection and tumorigenesis.63 Additionally, MSCs are readily available and can be obtained through minimally invasive procedures. To assess TM function following implantation, tonography and imaging techniques based on optical coherence tomography (OCT) would provide valuable insights.64, 65, 66 Further preclinical and clinical studies are required to enhance our understanding of stem cell therapy for glaucoma.
5. Applications of retina regeneration in ophthalmic disease treatment
The retina, a stratified sensory tissue, encompasses various cell types, such as retinal pigment epithelium (RPE) cells, photoreceptors, intermediate neurons, retinal ganglion cells (RGCs), and glial cells. This diverse array of cell types, as highlighted with RPE cells, photoreceptors, intermediate neurons, RGCs, and glial cells, holds promising potential for application in stem cell therapy.
Retinal degenerative diseases (RDDs) are a group of irreversible diseases characterized by the progressive degeneration of retinal cells. Examples of RDDs include diabetic retinopathy (DR), retinitis pigmentosa (RP), age-related macular degeneration (AMD), and glaucoma, which ultimately result in cell death. Imbalances in the retinal microenvironment contribute to retinal degeneration.67
Stem cell therapy is an effective approach to restore retinal function and treat RDDs, as the retina has limited regenerative capacity. Stem cells used in therapy can be sourced from RPC, ESC, iPSCs, MSC, and neural precursor cells (NPCs). Delivery strategies for stem cell therapy include encapsulated cell technology, polymer scaffolds, and retinal sheets. Various strategies are employed to avoid immune rejection, a common complication of cell transplantation.68 Using patient-specific autologous iPSC-derived cells is an ideal approach to achieve host-recipient match.69 However, the process of reprogramming and testing can be time-consuming and costly for personalized medicine. As an alternative, generating pools of HLA-matched lines is being actively pursued. Finding people that are homozygous for the human leukocyte antigen (HLA) alleles and creating certain induced pluripotent stem cells (iPSC) lines that may match a sizable fraction of the transplant recipient population are the two steps in the procedure.70,71 According to a recent study, nearly 40% of the Korean population is represented by the most prominent 10 HLA-homozygous iPSC lines.72 A different strategy is to introduce HLA-E genes at the B2M locus73 and knock down both alleles of β2-microglobulin (β2M)74 to create HLA-engineered lines. The immune system does not perceive these cells as allogeneic. As a result, they might be a good source for general donor cells.
5.1. Application of retinal pigment epithelium (RPE) replacement in ophthalmic disease treatment
The pigment cells known as RPE cells are in charge of carrying nutrients from the circulatory system to photoreceptors and breaking down decomposing disks from the photoreceptors’ outer segment.75
Graft rejection poses a significant concern for allogeneic transplants, and achieving major histocompatibility complex (MHC) matching is crucial for successful RPE transplantation. Studies have demonstrated that chronic rejection is mediated through the expression of MHC II.76 Additionally, the immunologic response has been found to be influenced by the number of transplanted cells, which increases over time.77,78 In vivo experiments with MHC-matched animal models have shown no signs of rejection in iPSC-derived RPE allografts, even without the use of immunosuppression. Conversely, MHC-mismatched models exhibited immune attacks around the graft and resulting damage to the retinal tissue.79 Studies conducted in vitro have shown that HLA-matched iPSC-derived RPE cells taken from HLA homozygous donors did not elicit a response from human T cells80
5.1.1. Regenerative treatment for age-related macular degeneration (AMD)
AMD is a degenerative disease that leads to blindness and is primarily caused by the loss or dysfunction of RPE. The absence of RPE results in the degeneration of a majority of the photoreceptors located above, resulting in a significant and progressive loss of vision. Results from preclinical and clinical studies suggest that RPE replacement techniques may be able to impede the disease’s progression and restore vision. While attempts have been made to transplant primary RPE in humans, the outcomes have been inconsistent in terms of graft survival and improvement of visual acuity.81 Utilizing stem cell derived cells offers significant advantages, such as an almost limitless cell supply and the ability to control their differentiation to guarantee optimal safety and potency prior to transplantation.
To find out if transplanting hESC-derived RPE into the subretinal space is safe and tolerable, numerous prospective clinical investigations were carried out.82, 83, 84, 85 These studies have reported improvements in BCVA, indicating promising early results for the use of transplanted hESC-RPE cells in alleviating AMD. In a phase I clinical study, patients with dry AMD showed an improvement in vision from 21 ETDRS letters to 28 following subretinal transplantation of hESC-derived RPE. Additionally, within 4 months, the hESC-derived RPE cells showed no evidence of hyperproliferation, tumorigenicity or apparent rejection.86 In another study, a highly mismatched donor RPE monolayer, implanted subretinally into a highly degenerate retina, demonstrated long-term survival and function for 2 years. The study also provided evidence of limited immunogenicity for the allogeneic hESC-RPE cells.87 It is thought that a very modest number of CD8 cytotoxic T cells in the implant location let these highly mismatch RPE cells survive and avoid being destroyed. In addition to the subretinal injection of hRPE, Kashani et al. have invented a new clinical-grade retinal implant composed of hESC-derived RPE that is cultivated on an ultrathin, synthetic parylene substrate designed to mimic Bruch’s membrane.88 Results at 1 year indicate that the implant is safe and well tolerated in individuals with advanced dry AMD.89 Regarding cell delivery methods, the injection of RPE cell suspension has a drawback because it only distributes a small number of cells over a damaged basal lamina, with unpredictable results.90,91 An alternative technique to the injection of RPE cell suspension is the transplantation of RPE-choroid sheets. Although there is a significant chance of postoperative complications with this approach, it provides a consistent cell sheet of polarized RPE on its basal lamina. Christiane and colleagues assessed the outcome of the two RPE transplantation methods.92 Their findings demonstrated comparable anatomical and functional outcomes for both techniques.
5.1.2. Regenerative treatment for Stargardt’s disease (STGD1)
Stargardt’s disease (STGD1) is one of the most common hereditary forms of juvenile macular degeneration. Initially, patients’ central vision is impaired. As the lipofuscin in apical RPE cells accumulate, the patients reported diminished vision, which results in secondary choroidal neovascularization and legal blindness.93
In order to evaluate the long-term safety and effectiveness of subretinal injection of hESC-RPE cell suspension in the early stage of STGD1, many clinical trials were carried out,94, 95, 96 in which none of the patients experienced adverse reactions systemically or locally. However, changes of long-term visual function were mixed. Another study conducted a detailed analysis of retinal anatomy and function using microperimetry and spectral-domain OCT.97 While no evidence of uncontrolled growth or inflammatory responses was found in any of the 12 participants, microperimetry indicated no benefits at 12 months for any of them. There was a possibility of injury since one person taking the maximum dose experienced localized retinal thinning and reduced sensitivity in the hyperpigmented region. Therefore, it is essential to consider the careful implementation of protective treatments following a longer duration of observation due to the slow rate of progressive degeneration in RDDs. In addition to hESCs, MSCs are also utilized for the treatment of STGD1. A phase II study involved the suprachoroidal implantation of ADMSCs in four patients. There were no signs of systemic or ocular complications, and all STGD1 patients showed improvements in visual field, and visual acuity during the six-month follow-up.98 However, the study’s patient population size was insufficient to permit a conclusive statistical analysis.
5.2. Applications of photoreceptor replacement in ophthalmic disease treatment
Photoreceptor replacement is practical since it is a unidirectional sensory neuron that requires just a single, brief synaptic connection, which might be produced from numerous sources such as RPCs, ESCs, iPSCs, and so on.
Located within the inner layer of the optic cup, RPCs are a type of NPC,99 possessing a similar proliferative capacity as stem cells. The specific properties of stem cells, including proliferation and differentiation, make RPC transplantation a promising approach for treating retinal diseases.
Researchers have discovered that co-culturing RPCs with hESC-RPE cells leads to an increased survival rate of RPCs in vitro.100 This may be attributed to the high levels of pigment epithelium-derived factor (PEDF) secreted by polarized RPE cells.101 These findings provide additional evidence supporting the idea that co-transplantation of integrated RPE and photoreceptor layers can be an effective method to maximize curative outcomes.
Single pigmented ciliary margin cells have the potential to differentiate into various retinal-specific cell types, including rod photoreceptors, bipolar neurons, and Müller glia. This indicates that these cells could serve as a viable substrate for retinal regeneration.102
5.2.1. Regenerative treatment for retinitis pigmentosa (RP)
RP encompasses a range of inherited diseases characterized by problems adjusting to darkness and night blindness in adolescence, loss of the mid-peripheral visual field in early adulthood, and eventual loss of central vision as a result of severe rod and cone photoreceptor degeneration.103 RP is a prevalent hereditary retinal disease, affecting approximately 1 in 4000 individuals.103
The first clinical trial to investigate the long-term safety of transplanting human fetal neuroretinal cells into RP patients was conducted by Taraprasad and colleagues in 1999.104 The study reported no complications in the host eyes. While initial follow-up showed improved light sensitivity, this improvement diminished between 3 and 13 months. In a similar trial, Liu et al. observed significant improvement in visual acuity for five out of eight patients between 2 and 6 months post-RPCs injection, but this improvement was not maintained at the 12-month mark.105 Another clinical trial demonstrated visual acuity score improvements for 50% of RP patients treated with neural RPC layers and RPE transplantation,106 providing evidence of the safety and benefits of retinal implants. Using the questionnaire, Rubens et al. examined the quality of life of RP patients who underwent intravitreal injection of autologous bone marrow MSCs (ABMSCs). At the three-month mark, they observed a statistically significant increase in the patients’ quality of life, but this improvement did not last at the 12-month mark.107 Administration of ADMSCs did not result in significant benefits and was associated with some ocular complications such as choroidal neovascular membrane and epiretinal membrane.108 Additionally, a preliminary study investigating intravitreal administration of autologous bone marrow CD34+ cells demonstrated short-term safety, but its efficacy remains inconclusive.109 Aekkachai et al. reported improvements in BCVA after intravitreal injection of bone marrow-derived mesenchymal stem cells (BM-MSCs) in participants with advanced RP, although the effects returned to baseline at 12 months.110 Another study reported infusion of human umbilical cord mesenchymal stem cells (UCMSCs) through the dorsal hand vein resulted in maintained or improved visual acuity for 81.3% of RP patients for 12 months.111 A prospective phase-III clinical study evaluating the efficacy of subtenon space transplantation of Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) enrolled 34 eyes from 32 RP patients. The study showed improvements in mean outer retinal thickness, horizontal ellipsoid zone width, and BCVA after 6 months, which remained stable at 1-year follow-up.112 Ongoing research is investigating the use of NPC-derived astrocytes (NCT04284293) and NPC-derived RPE (NCT03073733 and NCT02320812) for the treatment of RP.100
Despite promising results, one limitation of these studies is the challenge of obtaining sufficient human fetal retina donor tissue to treat large clinical populations.
5.2.2. Regenerative treatment for diabetic retinopathy (DR)
DR, a common complication of diabetes, is the main factor contributing to avoidable blindness in people in their working years.113 DR is classified as either proliferative or non-proliferative based on its stage of development.114 During the early non-proliferative stage, hyperglycemia damages the blood-retinal barrier, resulting in blood leakage. These changes initiate ischemic alterations in the surrounding retina. With the progression of hypoperfusion in the retinal capillary bed, proliferative diabetic retinopathy occurs. This condition is regarded as an ineffective response to ischemia, characterized by the development of new vessels at the papilla, on the retina, and the iris.114 Eventually, the proliferation of new vessels induced by localized retinal ischemia can result in tractional retinal detachment and subsequent vision loss.
In a pilot clinical trial, researchers evaluated the efficacy and safety of intravenous ABMSCs.115 A total of 17 patients with either non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR) contributed 34 analyzed eyes. Throughout the 6-month follow-up period, there were no instances of severe complications. Additionally, the NPDR group exhibited reductions in macular thickness and a notable improvement in baseline BCVA, whereas the PDR group did not demonstrate such changes. ABMSCs may provide a secure and efficient therapeutic alternative for DR, with the optimal therapeutic window appearing to be during the NPDR.
5.3. Applications of ganglion cells replacement in ophthalmic disease treatment
5.3.1. Regenerative treatment for glaucoma
Glaucoma, a neurodegenerative disease that causes a gradual loss of RGC. Although lowering IOP is the standard therapy for glaucoma, some individuals may not obtain adequate outcomes with maximum dose.
One proposed method of glaucoma stem cell treatment is the secretion of neuro-trophic factors by stem cells, which can promote the survival of RGC in animal models.116, 117, 118 Furthermore, stem cells are being studied as a potential source of replacement for the injured RGC.119 Although several studies suggested safety and potential utility of MSC transplantation,117,120 further clinical trials are still needed to demonstrate visual function improvement of this advanced therapy. There is a distinct obstacle existing compared to RPE and photoreceptor cell replacement: in order for the transplants or replacements to be successful, the transplanted cell must not only integrate into the ganglion cell layer, but also differentiate into RGC-like cells and generate axons from the cell to the optic nerve. Although it does not apply to iPSC, it has been shown that RGC-like cells derived from ESC and MSC can migrate and integrate into the retina.
6. Challenges and future prospects
The stem cell based cell therapy has been clinically applied in the ophthalmology practice for nearly 20 years. However, there are still many challenges in clinical applications. Firstly, almost all of the aforementioned clinical trials have limited serious complications. However, it is important to note that the use of stem cells cannot be considered completely safe. A major adverse event in the United States involving intravitreal stem cell injections has sparked widespread discussion. At a stem cell facility, three patients experienced severe vision impairment after bilateral intravitreal injections of autologous ADMSCs.121 This incident underscores the urgent need for comprehensive regulatory oversight of stem cell therapies studied at research facilities for medical conditions. To address this, new legislation must be enacted to establish rules, prohibit for-profit human research, and ensure patient safety. Moreover, regardless of whether autologous or allogeneic stem cells are used, a crucial prerequisite for mass-producing stem cells is establishing quality design principles and implementing automated and controllable systems. These measures can enhance the repeatability and consistency of cell product manufacturing, leading to cost and risk reduction. Before being used in functional studies on animals or humans, tests are necessary to verify the purity and efficacy of the stem cells. Secondly, stem cell products readily induce immune responses after injection. Therefore, stem cells should be designed with immune evasion characteristics. For example, stem cells that have MHC genes knocked out and overexpress anti-phagocytic molecules are undetectable by MHC-mismatched hosts. However, evading immune regulation can increase the risk of tumor development. One possible solution to avoid uncontrolled proliferation is the addition of suicide genes. In addition, most advanced degenerative ophthalmopathy will experience multiple types of cell loss so the effectiveness of single cell type transplantation is limited. A potential solution to this challenge is multicellular transplantation. Organoid, a remarkable innovation, is a three-dimensional self-organizing structure derived from adult tissue stem cells or pluripotent stem cells. It possesses the ability to simulate the functions and structures of the original tissue and has been utilized in treating neurodegenerative disorders. For eyes, it has been reported that organoid is used to treat photoreceptor degenerative diseases through replacing damaged or lost photoreceptors by forming mature cones and rods.122
This paper proposes several potential directions for future research:
Optimize stem cell sourcing and differentiation: investigate additional sources of autologous or homologous stem cells, enhance the efficiency and quality of stem cell differentiation, and mitigate immune and tumor risks associated with allogenic stem cells.
Improve stem cell delivery and integration: explore biocompatible and biodegradable carrier materials, enhance the survival rate and accuracy of stem cell placement, and facilitate the integration and functional recovery of stem cells with host tissue.
Improve the timing and dosage of stem cell therapy: determine optimal timing and dosage for treating various ophthalmic diseases with stem cells, strike a balance between efficacy and safety, and prevent adverse consequences of over- or underuse of stem cell therapy.
Enhance the evaluation and monitoring of stem cell therapy: investigate objective and sensitive evaluation indicators for stem cell therapy, establish a long-term follow-up and monitoring system for stem cell treatment, and promptly identify and manage complications and failures associated with stem cell therapy.
Improve the norms and regulations of stem cell therapy: establish robust norms and regulations for stem cell therapy, ensure the quality and safety of such therapy, prevent its abuse and misuse, and safeguard the rights and interests of patients.
MSC therapy, particularly its ability to enhance M2 microglial polarization, has demonstrated the release of anti-inflammatory factors that alleviate inflammation and improve neuronal cell conditions. This approach has been successful in treating various neurological disorders, including stroke, Alzheimer’s disease, and spinal cord damage. Therefore, M2 microglial transplantation holds potential as an efficient and suitable treatment option for degenerative eye diseases, given its viability and benefits demonstrated in brain research.
7. Conclusions
As an innovative solution, stem cell therapy is anticipated to substitute for traditional approaches to treat serious ocular injuries and diseases. Significant efforts have been devoted to investigating promising types of stem cells, such as LSCs and iPSCs, to tackle a broad range of eye diseases. Owing to their distinctive properties, these stem cells demonstrate potential for use in various clinical contexts. Presumably, novel stem cell therapies will be introduced in clinical practice in the near future, offering not only facilitation of tissue regeneration but also attenuation of inflammation and neuroprotective effects through the secretion of exosomes, enhancing patient outcomes. This highlights the multi-mechanistic therapeutic potential of stem cell therapy and will represent a new phase in ocular medicine.
Study approval
Not Applicable.
Author contributions
QLF and KY conceived and designed the study; YFN searched references of the study; JFJ selected the references; YFN wrote the original paper; QLF and KY re-wrote the final paper and submitted the pape. All authors have read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81670833, 81870641, 82070939, 81300641). This research was also supported by the Zhejiang Province Key Research and Development Program (2019C03091, 2020C03035), as well as the Fundamental Research Funds of the Central Universities (2019QNA7026).
Editorship disclosure
Given their role as [Editor-in-Chief], Ke Yao had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Andrzej Grzybowski.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to all the peer reviewers and editors for their opinions and suggestions.
Contributor Information
Yifei Niu, Email: niuyifei@zju.edu.cn.
Junfeng Ji, Email: jijunfeng@zju.edu.cn.
Ke Yao, Email: xlren@zju.edu.cn.
Qiuli Fu, Email: 2313009@zju.edu.cn.
Abbreviations
- ABMSCs
-
autologous bone marrow MSCs
- ADMSCs
-
adipose-derived MSCs
- AMD
-
age-related macular degeneration
- AMT
-
amniotic membrane transplantation
- BM-MSCs
-
bone marrow-derived mesenchymal stem cells
- CECs
-
corneal endothelial cells
- cGVHD
-
chronic Graft-versus-Host Disease
- CLAU
-
conjunctival limbal autografting
- CLET
-
cultivated limbal epithelium transplantation
- DED
-
dry eye diseases
- DPSCs
-
dental pulp stem cells
- ESCs
-
embryonic stem cells
- HFSCs
-
hair follicle-derived stem cells
- IOP
-
intraocular pressure
- iPSCs
-
induced pluripotent stem cells
- LBs
-
lentoid bodies
- LECs
-
lens epithelial stem/progenitor cells
- LSCD
-
limbal stem cell deficiency
- LSCs
-
limbal stem cells
- MHC
-
major histocompatibility complex
- MSC-exo
-
exosomes derived from MSCs
- MSCs
-
mesenchymal stem cells
- NPCs
-
neural precursor cells
- NPDR
-
non-proliferative diabetic retinopathy
- OCT
-
optical coherence tomography
- OME
-
oral mucosal epithelium
- PDR
-
proliferative diabetic retinopathy
- PEDF
-
pigment epithelium-derived factor
- POAG
-
primary open-angle glaucoma
- RDDs
-
retinal degenerative diseases
- RGCs
-
retinal ganglion cells
- ROCK
-
rho-associated protein kinase
- RP
-
retinitis pigmentosa
- RPCs
-
retinal progenitor cells
- RPE
-
retinal pigment epithelium
- SLET
-
simple limbal epithelial transplantation
- STGD1
-
Stargardt’s disease
- TM
-
trabecular meshwork
- TMSCs
-
Trabecular meshwork stem cells
- UCMSCs
-
umbilical cord mesenchymal stem cells
- UiPSCs
-
urinary human induced pluripotent stem cells
- VAO
-
visual axis opacification
- WJ-MSCs
-
Wharton’s jelly-derived mesenchymal stem cells